Atom
(Redirected from Atoms)
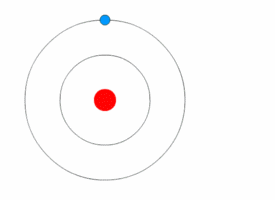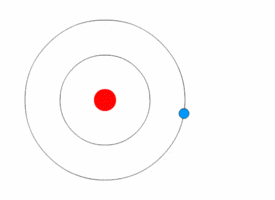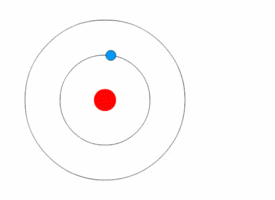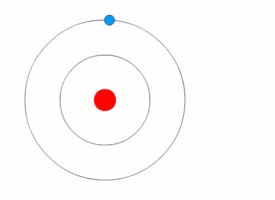
Atom is the fundamental building block of all matter. It is the smallest unit of an element that retains the chemical properties of that element. Atoms consist of a nucleus made of protons and neutrons, with electrons orbiting this nucleus. The study of atoms and their properties is a key part of chemistry and physics, providing the foundation for understanding the composition, structure, and reactions of all material substances.
Structure[edit | edit source]
The structure of an atom is characterized by its nucleus, which contains positively charged protons and neutrally charged neutrons, and the electron cloud that surrounds the nucleus. Electrons, which are negatively charged, orbit the nucleus at various energy levels or electron shells. The number of protons in the nucleus determines the atomic number of an element, which in turn defines the element's chemical properties. The total number of protons and neutrons in an atom's nucleus defines its atomic mass.
Behavior[edit | edit source]
Atoms can engage in various interactions, including chemical bonds to form molecules and compounds. The type of chemical bond (covalent, ionic, metallic, etc.) depends on the elements involved and their electron configurations. Atoms can gain or lose electrons, becoming ions (positively or negatively charged atoms) in the process. The behavior of atoms and their ability to form bonds is the basis for all chemical reactions.
History[edit | edit source]
The concept of the atom has evolved over centuries. Early philosophers like Democritus proposed the idea of indivisible particles as the building blocks of matter. However, it wasn't until the 19th century that scientists such as John Dalton developed the modern atomic theory, which provided a scientific basis for the concept of atoms. Subsequent discoveries, including the electron by J.J. Thomson, the nucleus by Ernest Rutherford, and the quantum mechanical model of the atom by Niels Bohr and others, have refined our understanding of atomic structure.
Quantum Mechanics[edit | edit source]
The behavior of electrons in atoms is governed by the principles of quantum mechanics, a branch of physics that describes the behavior of particles at the atomic and subatomic levels. Quantum mechanics introduces the concept of wave-particle duality, where electrons exhibit both particle-like and wave-like properties. The exact position of an electron in an atom cannot be determined; instead, quantum mechanics provides probabilities of finding an electron in certain regions around the nucleus.
Applications[edit | edit source]
Understanding atoms and their interactions is crucial for numerous scientific and technological fields. In chemistry, it enables the synthesis of new materials and compounds. In physics, the study of atomic and subatomic particles has led to the development of technologies such as nuclear energy and semiconductors. In biology, the understanding of atomic interactions is fundamental to the study of life at the molecular level.
See Also[edit | edit source]
Search WikiMD
Ad.Tired of being Overweight? Try W8MD's physician weight loss program.
Semaglutide (Ozempic / Wegovy and Tirzepatide (Mounjaro / Zepbound) available.
Advertise on WikiMD
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Translate this page: - East Asian
中文,
日本,
한국어,
South Asian
हिन्दी,
தமிழ்,
తెలుగు,
Urdu,
ಕನ್ನಡ,
Southeast Asian
Indonesian,
Vietnamese,
Thai,
မြန်မာဘာသာ,
বাংলা
European
español,
Deutsch,
français,
Greek,
português do Brasil,
polski,
română,
русский,
Nederlands,
norsk,
svenska,
suomi,
Italian
Middle Eastern & African
عربى,
Turkish,
Persian,
Hebrew,
Afrikaans,
isiZulu,
Kiswahili,
Other
Bulgarian,
Hungarian,
Czech,
Swedish,
മലയാളം,
मराठी,
ਪੰਜਾਬੀ,
ગુજરાતી,
Portuguese,
Ukrainian
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Contributors: Prab R. Tumpati, MD