Hierarchy of evidence
(Redirected from Levels of evidence)
Hierarchy of Evidence[edit | edit source]
Introduction[edit | edit source]
The hierarchy of evidence is a fundamental concept in evidence-based medicine, guiding clinicians in making informed decisions based on the quality and strength of research evidence.
Definition and Importance[edit | edit source]
The hierarchy of evidence is a system for classifying different types of medical research based on their reliability and validity. It helps in determining the most authoritative evidence for answering clinical questions, particularly regarding treatment and prevention.
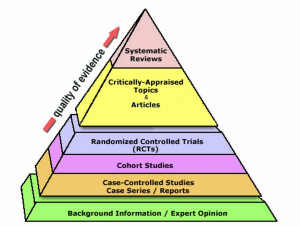
Levels of Evidence[edit | edit source]
Evidence in medical research is categorized into different levels, from highest to lowest, based on the methodological quality and potential for bias.
Systematic Reviews and Meta-Analyses[edit | edit source]
Systematic reviews and meta-analyses are at the top of the hierarchy. They synthesize data from multiple randomized controlled trials, offering comprehensive and high-quality evidence.
Randomized Controlled Trials[edit | edit source]
Randomized controlled trials (RCTs) are experiments where participants are randomly assigned to different groups to compare treatments. They are highly valued for their ability to minimize bias.
Cohort Studies[edit | edit source]
Cohort studies follow groups of people over time, observing the outcomes of a particular treatment or exposure. They are useful for studying the effects of less common treatments.
Case-Control Studies[edit | edit source]
Case-control studies compare patients with a condition (cases) to those without (controls) to identify potential causes or risk factors.
Case Reports and Series[edit | edit source]
Case reports and case series describe the experiences of single patients or groups. While offering valuable clinical insights, they are considered less reliable due to the absence of control groups.
Expert Opinion and Bench Research[edit | edit source]
Expert opinions, editorials, and bench research (pre-clinical studies) are at the lower end of the hierarchy, providing insights but often lacking rigorous scientific evaluation.
Applying the Hierarchy in Clinical Practice[edit | edit source]
Clinicians use the hierarchy to identify the best available evidence for patient care. It aids in understanding the strength of recommendations and tailoring treatments to individual patient needs.
Challenges and Criticisms[edit | edit source]
The hierarchy of evidence is not without its challenges and criticisms. Issues include the potential for bias in lower-level studies and the practical difficulties in conducting high-level studies like RCTs.
Conclusion[edit | edit source]
Understanding the hierarchy of evidence is essential for clinicians to practice evidence-based medicine effectively, ensuring the best possible patient outcomes.
References[edit | edit source]
- Evidence-Based Medicine Working Group. (1992). Evidence-based medicine: A new approach to teaching the practice of medicine. JAMA.
- Greenhalgh, T. (2014). How to read a paper: The basics of evidence-based medicine. Wiley-Blackwell.
- Guyatt, G. H., & Rennie, D. (2002). Users' guides to the medical literature: A manual for evidence-based clinical practice. AMA Press.
See Also[edit | edit source]
Categories[edit | edit source]
Search WikiMD
Ad.Tired of being Overweight? Try W8MD's NYC physician weight loss.
Semaglutide (Ozempic / Wegovy and Tirzepatide (Mounjaro / Zepbound) available. Call 718 946 5500.
Advertise on WikiMD
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Translate this page: - East Asian
中文,
日本,
한국어,
South Asian
हिन्दी,
தமிழ்,
తెలుగు,
Urdu,
ಕನ್ನಡ,
Southeast Asian
Indonesian,
Vietnamese,
Thai,
မြန်မာဘာသာ,
বাংলা
European
español,
Deutsch,
français,
Greek,
português do Brasil,
polski,
română,
русский,
Nederlands,
norsk,
svenska,
suomi,
Italian
Middle Eastern & African
عربى,
Turkish,
Persian,
Hebrew,
Afrikaans,
isiZulu,
Kiswahili,
Other
Bulgarian,
Hungarian,
Czech,
Swedish,
മലയാളം,
मराठी,
ਪੰਜਾਬੀ,
ગુજરાતી,
Portuguese,
Ukrainian
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Contributors: Kondreddy Naveen