Differential scanning calorimetry
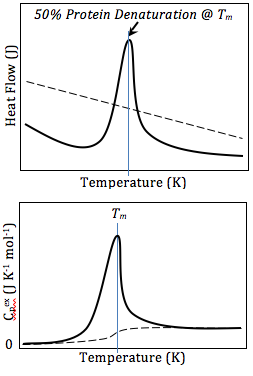
Differential Scanning Calorimetry (DSC) is a thermoanalytical technique in which the difference in the amount of heat required to increase the temperature of a sample and reference is measured as a function of temperature. Both the sample and reference are maintained at nearly the same temperature throughout the experiment. Generally, the temperature program for a DSC analysis is designed such that the sample holder temperature increases linearly as a function of time. The reference sample should have a well-defined heat capacity over the range of temperatures to be scanned.
Principle[edit | edit source]
The basic principle underlying DSC is the measurement of the heat flow to the sample and reference under controlled conditions of temperature. The difference in heat flow to the sample and reference, at any given temperature, represents the heat capacity and phase transitions occurring in the sample. This difference is recorded as a function of temperature or time and results in a DSC curve, which can provide qualitative and quantitative information about physical and chemical changes that involve endothermic or exothermic processes or changes in heat capacity.
Applications[edit | edit source]
DSC is widely used in chemical, pharmaceutical, and materials science fields for various applications, including:
- Determining the melting point, glass transition temperature, and crystallization temperature of polymers.
- Studying the thermal stability and composition of materials.
- Investigating the purity of compounds.
- Analyzing the effects of additives on the stability of materials.
- Examining phase transitions and reaction kinetics.
Types of DSC[edit | edit source]
There are mainly two types of DSC:
- Heat-flux DSC: In this type, both the sample and reference are placed on the same heat sink. The difference in heat flow to the sample and reference is measured.
- Power-compensated DSC: This type uses separate heaters for the sample and reference. The power applied to each heater is adjusted to maintain both at the same temperature. The difference in power is directly proportional to the heat flow difference.
Procedure[edit | edit source]
A typical DSC experiment involves placing a small amount of sample in a pan, which is then sealed and placed in the DSC instrument. The instrument is programmed to heat the sample at a controlled rate, often in an inert atmosphere to prevent oxidation or other reactions with air. As the sample is heated, it may undergo phase transitions, such as melting or crystallization, which result in a change in heat flow. These changes are detected by the DSC and recorded as a curve of heat flow versus temperature.
Data Analysis[edit | edit source]
The analysis of DSC data involves the interpretation of the DSC curves. Endothermic peaks, indicative of heat absorption, can represent melting or decomposition, while exothermic peaks, indicative of heat release, can represent crystallization or oxidation reactions. The area under these peaks is proportional to the enthalpy change associated with the transition.
Advantages and Limitations[edit | edit source]
DSC offers several advantages, including:
- Quick and easy determination of transition temperatures and enthalpies.
- Small sample size requirement.
- Applicability to a wide range of materials.
However, DSC also has limitations, such as:
- Difficulty in interpreting overlapping transitions.
- Sensitivity to atmospheric conditions.
- Requirement for reference materials with well-defined heat capacities.
Conclusion[edit | edit source]
Differential Scanning Calorimetry is a powerful analytical tool in the field of thermal analysis, providing valuable information about the physical and chemical properties of materials. Its ability to analyze a wide range of materials makes it indispensable in research and development in various scientific and industrial fields.
Search WikiMD
Ad.Tired of being Overweight? Try W8MD's physician weight loss program.
Semaglutide (Ozempic / Wegovy and Tirzepatide (Mounjaro / Zepbound) available.
Advertise on WikiMD
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Translate this page: - East Asian
中文,
日本,
한국어,
South Asian
हिन्दी,
தமிழ்,
తెలుగు,
Urdu,
ಕನ್ನಡ,
Southeast Asian
Indonesian,
Vietnamese,
Thai,
မြန်မာဘာသာ,
বাংলা
European
español,
Deutsch,
français,
Greek,
português do Brasil,
polski,
română,
русский,
Nederlands,
norsk,
svenska,
suomi,
Italian
Middle Eastern & African
عربى,
Turkish,
Persian,
Hebrew,
Afrikaans,
isiZulu,
Kiswahili,
Other
Bulgarian,
Hungarian,
Czech,
Swedish,
മലയാളം,
मराठी,
ਪੰਜਾਬੀ,
ગુજરાતી,
Portuguese,
Ukrainian
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Contributors: Prab R. Tumpati, MD