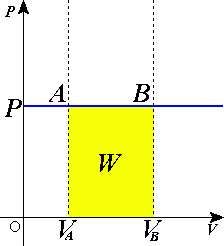
Isobaric process
An isobaric process is a thermodynamic process in which the pressure remains constant: ΔP = 0. The term "isobaric" comes from the Greek words "iso", meaning equal, and "baros", meaning weight. This process is significant in the study of thermodynamics, a branch of physics that deals with the relationships between heat, work, temperature, and energy.
In an isobaric process, since the pressure of the system is constant, any heat added or removed from the system will result in a change in the volume and temperature of the system. According to the first law of thermodynamics, the work done by the system in an isobaric process can be expressed as W = PΔV, where W is work, P is the constant pressure, and ΔV is the change in volume.
The concept of an isobaric process is widely applied in various scientific and engineering fields, such as in the design of heat engines and refrigeration cycles. For example, one of the steps in the Carnot cycle, which is a theoretical thermodynamic cycle proposed by Nicolas Léonard Sadi Carnot, is an isobaric expansion or compression.
In the context of the ideal gas law, which is a good approximation for the behavior of real gases under many conditions, an isobaric process follows the formula P = nRT/V, where P is the pressure, n is the number of moles of gas, R is the ideal gas constant, T is the temperature in Kelvin, and V is the volume. Since the pressure is constant, any change in the volume or temperature of the gas can be directly related to each other by the equation ΔT/ΔV = P/nR.
Understanding isobaric processes is crucial for the study of atmospheric sciences, including meteorology and climatology, as these processes are fundamental to the dynamics of the Earth's atmosphere. For instance, isobaric cooling and warming are key concepts in weather prediction and climate modeling.
Search WikiMD
Ad.Tired of being Overweight? Try W8MD's NYC physician weight loss.
Semaglutide (Ozempic / Wegovy and Tirzepatide (Mounjaro / Zepbound) available. Call 718 946 5500.
Advertise on WikiMD
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Translate this page: - East Asian
中文,
日本,
한국어,
South Asian
हिन्दी,
தமிழ்,
తెలుగు,
Urdu,
ಕನ್ನಡ,
Southeast Asian
Indonesian,
Vietnamese,
Thai,
မြန်မာဘာသာ,
বাংলা
European
español,
Deutsch,
français,
Greek,
português do Brasil,
polski,
română,
русский,
Nederlands,
norsk,
svenska,
suomi,
Italian
Middle Eastern & African
عربى,
Turkish,
Persian,
Hebrew,
Afrikaans,
isiZulu,
Kiswahili,
Other
Bulgarian,
Hungarian,
Czech,
Swedish,
മലയാളം,
मराठी,
ਪੰਜਾਬੀ,
ગુજરાતી,
Portuguese,
Ukrainian
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Contributors: Prab R. Tumpati, MD