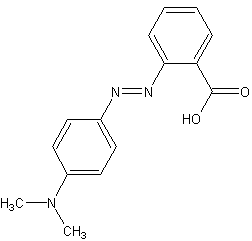
Methyl red
Methyl red (MR), also known as C.I. Acid Red 2, is an indicator dye that turns red in acidic solutions. It is a pH indicator used to identify the acidity of a solution. Methyl red is an azo dye, and it changes color at a pH of about 4.4 to 6.2, from red at pH 4.4 and below to yellow at pH 6.2 and above, with the transition range between these values appearing orange.
Chemistry[edit | edit source]
Methyl red is a chemical compound with the formula C15H15N3O2. It is part of the azo dye group, which are characterized by the presence of the functional group -N=N-, known as an azo group. In an acidic environment, methyl red offers a red color due to the presence of hydronium ions (H3O+) which stabilize the structure that absorbs at longer wavelengths. In neutral to basic conditions, the molecule exists in a form that absorbs at shorter wavelengths, thus appearing yellow.
Application[edit | edit source]
Methyl red is widely used in the laboratory as a pH indicator in titration experiments. It is particularly useful in acid-base titrations due to its clear and distinct color change. Besides its use in chemistry, methyl red is also employed in the microbiology field, specifically in the Methyl Red test, part of the IMViC tests, to identify bacteria that produce stable acid end products from glucose fermentation.
Methyl Red Test[edit | edit source]
The Methyl Red test is a procedure used in microbiology to detect the ability of microorganisms to perform mixed acids fermentation when supplied with glucose. The presence of acid end-products lowers the pH of the medium, and the addition of methyl red indicator to the culture turns the medium red, indicating a positive test. Organisms that utilize the butylene glycol pathway or other non-acidic pathways result in a negative test, with the medium turning yellow.
Safety[edit | edit source]
As with many azo dyes, there are safety considerations with methyl red. It should be handled with care in the laboratory, using appropriate personal protective equipment. Methyl red is considered to be a potential health hazard if ingested, inhaled, or comes into contact with skin and eyes.
Environmental Impact[edit | edit source]
The environmental impact of azo dyes, including methyl red, is a concern due to their synthetic nature and potential to release aromatic amines, some of which may be carcinogenic, upon degradation. Efforts are ongoing to develop more environmentally friendly alternatives and to improve the degradation processes of existing dyes to minimize their impact.
Methyl_red[edit | edit source]
Methyl red from crystal 3D balls
Crystals of Methyl red sodium salt
Preparation of Methyl Red
Search WikiMD
Ad.Tired of being Overweight? Try W8MD's physician weight loss program.
Semaglutide (Ozempic / Wegovy and Tirzepatide (Mounjaro / Zepbound) available.
Advertise on WikiMD
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Translate this page: - East Asian
中文,
日本,
한국어,
South Asian
हिन्दी,
தமிழ்,
తెలుగు,
Urdu,
ಕನ್ನಡ,
Southeast Asian
Indonesian,
Vietnamese,
Thai,
မြန်မာဘာသာ,
বাংলা
European
español,
Deutsch,
français,
Greek,
português do Brasil,
polski,
română,
русский,
Nederlands,
norsk,
svenska,
suomi,
Italian
Middle Eastern & African
عربى,
Turkish,
Persian,
Hebrew,
Afrikaans,
isiZulu,
Kiswahili,
Other
Bulgarian,
Hungarian,
Czech,
Swedish,
മലയാളം,
मराठी,
ਪੰਜਾਬੀ,
ગુજરાતી,
Portuguese,
Ukrainian
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Contributors: Prab R. Tumpati, MD