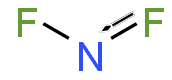
Nitrogen difluoride
Nitrogen difluoride[edit | edit source]
Nitrogen difluoride (NF2) is a chemical compound consisting of one nitrogen atom and two fluorine atoms. It is a radical species and is known for its role in various chemical reactions, particularly in the field of inorganic chemistry.
Structure and Properties[edit | edit source]
Nitrogen difluoride is a diatomic molecule with a bent molecular geometry. The presence of the unpaired electron on the nitrogen atom makes it a radical, which contributes to its high reactivity. The bond angle in NF2 is approximately 102 degrees, and the N-F bond length is about 1.35 Å.
The compound is typically found in the gas phase and is known for its instability. It can be generated through the reaction of nitrogen trifluoride (NF3) with atomic fluorine or through the photolysis of NF3.
Chemical Reactions[edit | edit source]
Nitrogen difluoride is involved in various chemical reactions due to its radical nature. It can participate in radical reactions, where it acts as an intermediate. NF2 can react with other radicals or molecules, leading to the formation of more stable compounds.
One notable reaction is its combination with other nitrogen-containing species to form nitrogen oxides or other nitrogen-fluorine compounds. The reactivity of NF2 makes it a subject of interest in the study of atmospheric chemistry and combustion processes.
Applications[edit | edit source]
While nitrogen difluoride itself is not widely used due to its instability, its derivatives and related compounds have applications in various fields. For example, nitrogen-fluorine compounds are used in the production of semiconductors and in the aerospace industry for their high energy content.
Safety and Handling[edit | edit source]
Due to its reactive nature, nitrogen difluoride must be handled with caution. It is typically studied under controlled laboratory conditions. Proper safety protocols, including the use of protective equipment and ventilation, are essential when working with NF2.
Related pages[edit | edit source]
Search WikiMD
Ad.Tired of being Overweight? Try W8MD's physician weight loss program.
Semaglutide (Ozempic / Wegovy and Tirzepatide (Mounjaro / Zepbound) available.
Advertise on WikiMD
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Translate this page: - East Asian
中文,
日本,
한국어,
South Asian
हिन्दी,
தமிழ்,
తెలుగు,
Urdu,
ಕನ್ನಡ,
Southeast Asian
Indonesian,
Vietnamese,
Thai,
မြန်မာဘာသာ,
বাংলা
European
español,
Deutsch,
français,
Greek,
português do Brasil,
polski,
română,
русский,
Nederlands,
norsk,
svenska,
suomi,
Italian
Middle Eastern & African
عربى,
Turkish,
Persian,
Hebrew,
Afrikaans,
isiZulu,
Kiswahili,
Other
Bulgarian,
Hungarian,
Czech,
Swedish,
മലയാളം,
मराठी,
ਪੰਜਾਬੀ,
ગુજરાતી,
Portuguese,
Ukrainian
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Contributors: Prab R. Tumpati, MD