Nuclear force
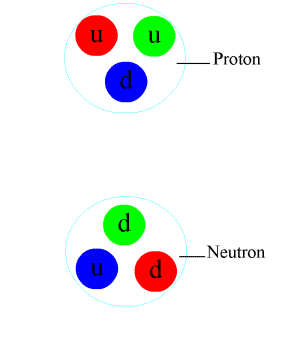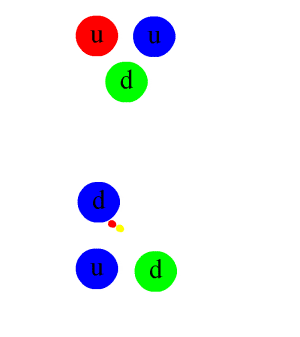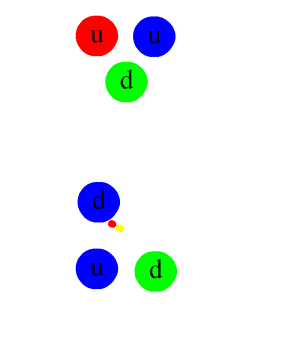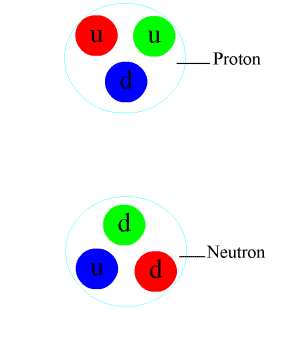
Nuclear force is a fundamental interaction responsible for the binding of protons and neutrons in an atomic nucleus. It is also known as the strong force or strong nuclear force. This force is one of the four fundamental forces of nature, alongside the gravitational force, electromagnetic force, and the weak nuclear force. The nuclear force plays a crucial role in the stability of matter and the structure of atoms, influencing the properties of elements and the variety of isotopes found in nature.
Overview[edit | edit source]
The nuclear force is characterized by its short range, acting effectively only over distances on the order of 1 to 3 femtometers (1 fm = 10^-15 meters). Unlike the gravitational and electromagnetic forces, which have infinite range and decrease in strength as the distance between objects increases, the nuclear force quickly drops off beyond its effective range. This force is attractive and becomes repulsive only at very short distances, less than about 0.7 fm, a phenomenon that helps to keep the nuclear particles (protons and neutrons) at an optimal distance from each other within the nucleus.
Properties[edit | edit source]
The strength of the nuclear force is approximately 10^38 times stronger than the gravitational force, making it the strongest force over subatomic distances. However, its influence is confined within the nucleus due to its short range. The nuclear force is charge-independent, meaning it acts equally on protons and neutrons. This property is crucial for the stability of the nucleus, especially in heavier elements where the repulsive electromagnetic force between protons would otherwise split the nucleus apart.
The nuclear force is mediated by particles called mesons, primarily the pions (π mesons), which are exchanged between nucleons (protons and neutrons). This exchange can be thought of as the physical mechanism by which the strong force operates, analogous to the exchange of photons in electromagnetic interactions.
Theoretical Framework[edit | edit source]
The underlying theory describing the nuclear force is quantum chromodynamics (QCD), which is a part of the Standard Model of particle physics. QCD deals with the interactions between quarks and gluons, the fundamental constituents of protons and neutrons. The strong force arises from the color charge of quarks and gluons, and it acts to confine quarks within nucleons and nucleons within the nucleus.
Role in Nuclear Reactions[edit | edit source]
The nuclear force is responsible for the phenomena of nuclear fusion and nuclear fission, which are the processes that power stars, including the Sun, and nuclear reactors, respectively. In nuclear fusion, the strong force allows nuclei to overcome their electromagnetic repulsion and combine to form heavier nuclei, releasing vast amounts of energy in the process. In nuclear fission, the nucleus of an atom splits into two or more smaller nuclei, again releasing energy due to the rearrangement of nuclear forces.
Implications and Applications[edit | edit source]
Understanding the nuclear force has led to significant advancements in various fields, including nuclear physics, nuclear medicine, and energy production. Research in nuclear physics has paved the way for the development of nuclear reactors, which are a source of power in many parts of the world. In medicine, nuclear technologies are used in diagnostic imaging and cancer treatment.
See Also[edit | edit source]
Search WikiMD
Ad.Tired of being Overweight? Try W8MD's physician weight loss program.
Semaglutide (Ozempic / Wegovy and Tirzepatide (Mounjaro / Zepbound) available.
Advertise on WikiMD
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Translate this page: - East Asian
中文,
日本,
한국어,
South Asian
हिन्दी,
தமிழ்,
తెలుగు,
Urdu,
ಕನ್ನಡ,
Southeast Asian
Indonesian,
Vietnamese,
Thai,
မြန်မာဘာသာ,
বাংলা
European
español,
Deutsch,
français,
Greek,
português do Brasil,
polski,
română,
русский,
Nederlands,
norsk,
svenska,
suomi,
Italian
Middle Eastern & African
عربى,
Turkish,
Persian,
Hebrew,
Afrikaans,
isiZulu,
Kiswahili,
Other
Bulgarian,
Hungarian,
Czech,
Swedish,
മലയാളം,
मराठी,
ਪੰਜਾਬੀ,
ગુજરાતી,
Portuguese,
Ukrainian
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Contributors: Prab R. Tumpati, MD