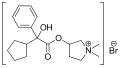
Glycopyrronium bromide
Glycopyrronium bromide is a medication that belongs to a class of drugs known as anticholinergics or antimuscarinics. It is primarily used to treat conditions that involve the overactivity of the muscarinic acetylcholine receptors. These conditions include chronic obstructive pulmonary disease (COPD), excessive sweating (hyperhidrosis), and saliva control issues in patients with neurological conditions. Glycopyrronium bromide works by blocking the action of acetylcholine, a chemical messenger involved in the transmission of nerve impulses, thereby reducing the secretion of bodily fluids and relaxing smooth muscles.
Medical Uses[edit]
Glycopyrronium bromide is utilized in various medical scenarios, each leveraging its anticholinergic properties to provide relief from different symptoms:
- Chronic Obstructive Pulmonary Disease (COPD): It is used as a maintenance treatment to alleviate symptoms of COPD, including chronic bronchitis and emphysema. It helps by relaxing the muscles around the airways, making breathing easier.
- Hyperhidrosis: For individuals suffering from excessive sweating, glycopyrronium bromide can significantly reduce sweat production.
- Saliva Control: In patients with neurological conditions that lead to difficulty controlling saliva, this medication can help reduce saliva production.
Pharmacology[edit]
Glycopyrronium bromide acts by competitively inhibiting the muscarinic acetylcholine receptors. This inhibition prevents acetylcholine from binding to these receptors, which are found in various tissues in the body, including the lungs, gastrointestinal tract, and sweat glands. By blocking the action of acetylcholine, glycopyrronium bromide reduces secretions and relaxes smooth muscles, thereby exerting its therapeutic effects.
Mechanism of Action[edit]
The primary mechanism of action of glycopyrronium bromide is its competitive antagonism of the muscarinic acetylcholine receptors (M1, M2, and M3 subtypes). This antagonism leads to a decrease in the effects of acetylcholine, a neurotransmitter that plays a significant role in the autonomic nervous system. In the lungs, this results in bronchodilation, which helps to improve airflow and relieve symptoms of COPD. In the sweat glands, it reduces sweat production, and in the salivary glands, it decreases saliva output.
Side Effects[edit]
Like all medications, glycopyrronium bromide can cause side effects, although not everyone experiences them. Common side effects include dry mouth, constipation, urinary retention, and blurred vision. These are typically mild and often resolve with continued use of the medication. However, due to its anticholinergic effects, it can also cause more serious side effects such as difficulty urinating, glaucoma exacerbation, and confusion in elderly patients. Patients are advised to discuss any side effects or concerns with their healthcare provider.
Administration[edit]
Glycopyrronium bromide is available in various forms, including oral tablets, inhalation powder, and topical formulations. The mode of administration depends on the condition being treated. For COPD, it is commonly prescribed as an inhalation powder to be used with a specific inhaler device. For hyperhidrosis, it may be prescribed in oral form or as a topical solution. The dosage and frequency of administration are determined by the healthcare provider based on the patient's condition and response to treatment.
Precautions[edit]
Patients with certain medical conditions should use glycopyrronium bromide with caution. These conditions include glaucoma, urinary retention, and enlarged prostate. It is also important for patients to inform their healthcare provider of all medications they are taking, as glycopyrronium bromide can interact with other drugs, potentially leading to adverse effects.
-
Glycopyrronium bromide
Ad. Transform your life with W8MD's Budget GLP-1 injections from $75


W8MD offers a medical weight loss program to lose weight in Philadelphia. Our physician-supervised medical weight loss provides:
- Weight loss injections in NYC (generic and brand names):
- Zepbound / Mounjaro, Wegovy / Ozempic, Saxenda
- Most insurances accepted or discounted self-pay rates. We will obtain insurance prior authorizations if needed.
- Generic GLP1 weight loss injections from $75 for the starting dose.
- Also offer prescription weight loss medications including Phentermine, Qsymia, Diethylpropion, Contrave etc.
NYC weight loss doctor appointmentsNYC weight loss doctor appointments
Start your NYC weight loss journey today at our NYC medical weight loss and Philadelphia medical weight loss clinics.
- Call 718-946-5500 to lose weight in NYC or for medical weight loss in Philadelphia 215-676-2334.
- Tags:NYC medical weight loss, Philadelphia lose weight Zepbound NYC, Budget GLP1 weight loss injections, Wegovy Philadelphia, Wegovy NYC, Philadelphia medical weight loss, Brookly weight loss and Wegovy NYC
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Translate this page: - East Asian
中文,
日本,
한국어,
South Asian
हिन्दी,
தமிழ்,
తెలుగు,
Urdu,
ಕನ್ನಡ,
Southeast Asian
Indonesian,
Vietnamese,
Thai,
မြန်မာဘာသာ,
বাংলা
European
español,
Deutsch,
français,
Greek,
português do Brasil,
polski,
română,
русский,
Nederlands,
norsk,
svenska,
suomi,
Italian
Middle Eastern & African
عربى,
Turkish,
Persian,
Hebrew,
Afrikaans,
isiZulu,
Kiswahili,
Other
Bulgarian,
Hungarian,
Czech,
Swedish,
മലയാളം,
मराठी,
ਪੰਜਾਬੀ,
ગુજરાતી,
Portuguese,
Ukrainian