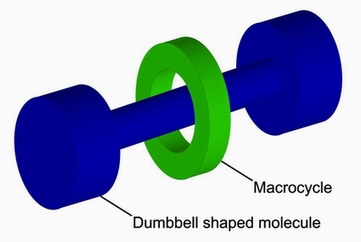
Rotaxane
Rotaxane is a type of molecular machine or supramolecular assembly consisting of a "dumbbell-shaped" molecule that is threaded through a macrocycle (a large, ring-shaped molecule). The macrocycle is able to move along the dumbbell but is prevented from dissociating completely due to the bulky end groups of the dumbbell-shaped molecule. This unique structure allows rotaxanes to exhibit mechanical motion at the molecular level, making them of great interest in the field of nanotechnology and molecular electronics. The name "rotaxane" is derived from the Latin words rota (wheel) and axis (axle), reflecting the wheel-on-axle structure of these compounds. The synthesis of rotaxanes involves the careful design of both the macrocycle and the dumbbell components to ensure that the macrocycle can thread onto the dumbbell and remain trapped by the bulky end groups. This process often utilizes supramolecular chemistry principles such as non-covalent interactions including hydrogen bonding, metal coordination, hydrophobic forces, and electrostatic interactions. Rotaxanes have been explored for various applications, including as molecular switches, molecular motors, and components in molecular computers. Their ability to undergo controlled and reversible mechanical movements in response to external stimuli (such as changes in pH, light, or electrical potential) makes them promising materials for the development of responsive systems at the nanoscale. One of the most well-known applications of rotaxanes is in the development of molecular shuttles, where the macrocycle can move between different positions on the dumbbell in a controlled manner. This shuttling motion has been harnessed for the creation of molecular switches that can toggle between distinct states, offering potential for use in molecular electronic devices and data storage systems. The study of rotaxanes also contributes to our understanding of mechanical bonds, a type of chemical bonding that arises from the mechanical interlocking of molecules rather than the sharing or transfer of electrons. This area of chemistry challenges traditional concepts of molecular structure and bonding, providing new insights into the design and synthesis of complex molecular systems.
Search WikiMD
Ad.Tired of being Overweight? Try W8MD's physician weight loss program.
Semaglutide (Ozempic / Wegovy and Tirzepatide (Mounjaro / Zepbound) available.
Advertise on WikiMD
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Translate this page: - East Asian
中文,
日本,
한국어,
South Asian
हिन्दी,
தமிழ்,
తెలుగు,
Urdu,
ಕನ್ನಡ,
Southeast Asian
Indonesian,
Vietnamese,
Thai,
မြန်မာဘာသာ,
বাংলা
European
español,
Deutsch,
français,
Greek,
português do Brasil,
polski,
română,
русский,
Nederlands,
norsk,
svenska,
suomi,
Italian
Middle Eastern & African
عربى,
Turkish,
Persian,
Hebrew,
Afrikaans,
isiZulu,
Kiswahili,
Other
Bulgarian,
Hungarian,
Czech,
Swedish,
മലയാളം,
मराठी,
ਪੰਜਾਬੀ,
ગુજરાતી,
Portuguese,
Ukrainian
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Contributors: Prab R. Tumpati, MD