ATP synthase
ATP synthase is a complex enzyme that plays a critical role in bioenergetics, the process by which living cells produce energy. Found in the mitochondria of eukaryotes and in the plasmids of prokaryotes, ATP synthase is essential for ATP (adenosine triphosphate) production, which is a universal energy currency in all living organisms. This enzyme facilitates the conversion of adenosine diphosphate (ADP) and inorganic phosphate (Pi) into ATP through a process known as oxidative phosphorylation in eukaryotes and photophosphorylation in photosynthetic organisms.
Structure and Function[edit | edit source]
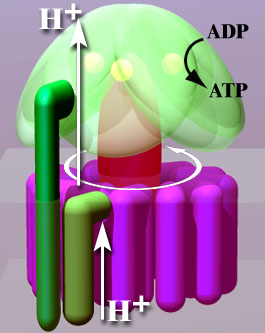
ATP synthase consists of two main components: F0 and F1. The F0 portion is embedded in the mitochondrial membrane (or plasmid membrane in prokaryotes) and functions as a proton channel. The F1 component protrudes into the mitochondrial matrix (or cytoplasm in prokaryotes) and is responsible for the synthesis of ATP from ADP and Pi. The flow of protons (H+) through the F0 unit drives the rotation of the F1 unit, which in turn catalyzes the synthesis of ATP.
The mechanism by which ATP synthase operates is often compared to a rotary engine. The proton gradient established by the electron transport chain across the mitochondrial or plasmid membrane provides the energy necessary for the rotation of the ATP synthase complex. This rotation leads to conformational changes in the F1 unit, enabling the binding of ADP and Pi, and the subsequent release of ATP.
Biological Importance[edit | edit source]
ATP synthase is vital for the survival of all living organisms because it is the primary source of ATP, which is used as an energy currency. ATP is necessary for numerous cellular processes, including muscle contraction, nerve impulse propagation, and chemical synthesis. Without ATP synthase, cells would not be able to produce ATP efficiently, leading to energy deficiency and impaired cellular function.
Genetic and Medical Significance[edit | edit source]
Mutations in the genes encoding ATP synthase components can lead to a variety of genetic disorders. These conditions often affect the nervous system and muscular system, as these tissues have high energy demands. Understanding the structure and function of ATP synthase is also crucial for the development of certain medications and treatments for diseases related to mitochondrial dysfunction.
Research and Applications[edit | edit source]
Research on ATP synthase has led to significant scientific advancements, including the development of drugs that target this enzyme to treat conditions such as ischemia and heart failure. Additionally, understanding the mechanism of ATP synthase has implications for the study of aging and metabolic diseases, as well as for the development of novel therapeutic strategies.
This article is a biochemistry stub. You can help WikiMD by expanding it!
Search WikiMD
Ad.Tired of being Overweight? Try W8MD's physician weight loss program.
Semaglutide (Ozempic / Wegovy and Tirzepatide (Mounjaro / Zepbound) available.
Advertise on WikiMD
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Translate this page: - East Asian
中文,
日本,
한국어,
South Asian
हिन्दी,
தமிழ்,
తెలుగు,
Urdu,
ಕನ್ನಡ,
Southeast Asian
Indonesian,
Vietnamese,
Thai,
မြန်မာဘာသာ,
বাংলা
European
español,
Deutsch,
français,
Greek,
português do Brasil,
polski,
română,
русский,
Nederlands,
norsk,
svenska,
suomi,
Italian
Middle Eastern & African
عربى,
Turkish,
Persian,
Hebrew,
Afrikaans,
isiZulu,
Kiswahili,
Other
Bulgarian,
Hungarian,
Czech,
Swedish,
മലയാളം,
मराठी,
ਪੰਜਾਬੀ,
ગુજરાતી,
Portuguese,
Ukrainian
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Contributors: Prab R. Tumpati, MD