Heteroazeotrope
Heteroazeotrope
A heteroazeotrope is a type of azeotrope where the components of the mixture are partially immiscible, meaning they form two separate liquid phases in equilibrium with a single vapor phase. This phenomenon occurs in certain binary or multicomponent mixtures and is characterized by a constant boiling point, which is different from the boiling points of the individual components.
Formation of Heteroazeotropes[edit | edit source]
Heteroazeotropes form when the intermolecular forces between the different components of the mixture are such that they prefer to separate into distinct liquid phases. This separation can be due to differences in polarity, hydrogen bonding, or other molecular interactions. When the mixture is heated, the vapor phase that forms has a constant composition, and the boiling point of the mixture remains constant.
Examples of Heteroazeotropes[edit | edit source]
One common example of a heteroazeotrope is the mixture of water and benzene. When these two liquids are mixed, they form two separate liquid layers due to their immiscibility. Upon heating, the vapor phase that forms has a constant composition and boils at a temperature that is different from the boiling points of pure water or pure benzene.
Applications[edit | edit source]
Heteroazeotropes are important in various industrial processes, particularly in distillation and separation techniques. They are used in the chemical industry to separate components of a mixture that are difficult to separate by simple distillation. Understanding the behavior of heteroazeotropes is crucial for designing efficient separation processes.
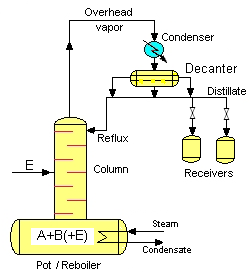
Distillation of Heteroazeotropes[edit | edit source]
The distillation of heteroazeotropes involves the use of techniques such as azeotropic distillation or extractive distillation. These methods take advantage of the unique boiling point and phase behavior of heteroazeotropes to achieve separation. In azeotropic distillation, an additional component called an entrainer is added to the mixture to form a new azeotrope with one of the original components, facilitating separation.
See Also[edit | edit source]
References[edit | edit source]
External Links[edit | edit source]
Search WikiMD
Ad.Tired of being Overweight? Try W8MD's physician weight loss program.
Semaglutide (Ozempic / Wegovy and Tirzepatide (Mounjaro / Zepbound) available.
Advertise on WikiMD
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Translate this page: - East Asian
中文,
日本,
한국어,
South Asian
हिन्दी,
தமிழ்,
తెలుగు,
Urdu,
ಕನ್ನಡ,
Southeast Asian
Indonesian,
Vietnamese,
Thai,
မြန်မာဘာသာ,
বাংলা
European
español,
Deutsch,
français,
Greek,
português do Brasil,
polski,
română,
русский,
Nederlands,
norsk,
svenska,
suomi,
Italian
Middle Eastern & African
عربى,
Turkish,
Persian,
Hebrew,
Afrikaans,
isiZulu,
Kiswahili,
Other
Bulgarian,
Hungarian,
Czech,
Swedish,
മലയാളം,
मराठी,
ਪੰਜਾਬੀ,
ગુજરાતી,
Portuguese,
Ukrainian
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates Wikipedia, licensed under CC BY SA or similar.
Contributors: Prab R. Tumpati, MD