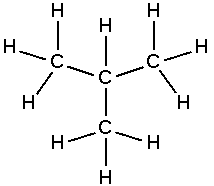
Isobutane
Isobutane, also known as methylpropane, is a hydrocarbon of the alkane series. It is a gas at room temperature and is used in a variety of applications, from refrigeration to the production of synthetic rubber.
Chemical Properties[edit | edit source]
Isobutane is a branched-chain alkane with four carbon atoms. Its chemical formula is C4H10. It is an isomer of butane, meaning it has the same number of atoms, but they are arranged differently. This gives isobutane different physical and chemical properties from butane.
Uses[edit | edit source]
Isobutane is used in a variety of applications. It is commonly used as a refrigerant due to its low boiling point. It is also used as a propellant in aerosol cans. In the petrochemical industry, isobutane is used in the production of isobutylene, which is a key ingredient in the production of synthetic rubber and other plastic materials.
Safety[edit | edit source]
Like other alkanes, isobutane is highly flammable. It can form explosive mixtures with air and should be handled with care. It is also a potent greenhouse gas, with a global warming potential much higher than that of carbon dioxide.
See Also[edit | edit source]
Search WikiMD
Ad.Tired of being Overweight? Try W8MD's physician weight loss program.
Semaglutide (Ozempic / Wegovy and Tirzepatide (Mounjaro / Zepbound) available.
Advertise on WikiMD
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Translate this page: - East Asian
中文,
日本,
한국어,
South Asian
हिन्दी,
தமிழ்,
తెలుగు,
Urdu,
ಕನ್ನಡ,
Southeast Asian
Indonesian,
Vietnamese,
Thai,
မြန်မာဘာသာ,
বাংলা
European
español,
Deutsch,
français,
Greek,
português do Brasil,
polski,
română,
русский,
Nederlands,
norsk,
svenska,
suomi,
Italian
Middle Eastern & African
عربى,
Turkish,
Persian,
Hebrew,
Afrikaans,
isiZulu,
Kiswahili,
Other
Bulgarian,
Hungarian,
Czech,
Swedish,
മലയാളം,
मराठी,
ਪੰਜਾਬੀ,
ગુજરાતી,
Portuguese,
Ukrainian
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Contributors: Prab R. Tumpati, MD