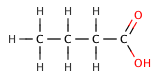Acid strength
(Redirected from Strong acid)
Overview of acid strength in chemistry
Acid Strength[edit | edit source]
Acid strength refers to the tendency of an acid to dissociate into a hydrogen ion (H⁺) and a conjugate base. The strength of an acid is determined by its ability to donate protons to a base, typically water, in an aqueous solution. Strong acids completely dissociate in water, while weak acids only partially dissociate.
Strong Acids[edit | edit source]
Strong acids are characterized by their complete ionization in water. This means that when a strong acid is dissolved in water, it releases all of its hydrogen ions into the solution. Common examples of strong acids include hydrochloric acid (HCl), sulfuric acid (H₂SO₄), and nitric acid (HNO₃). These acids have a high acid dissociation constant (Kₐ), indicating their strong ability to donate protons.
Weak Acids[edit | edit source]
Weak acids only partially dissociate in water, meaning that only a fraction of the acid molecules release hydrogen ions into the solution. This partial dissociation is represented by an equilibrium between the undissociated acid and the ions produced. Examples of weak acids include acetic acid (CH₃COOH) and formic acid (HCOOH). Weak acids have a lower acid dissociation constant compared to strong acids.
Factors Affecting Acid Strength[edit | edit source]
Several factors influence the strength of an acid:
Electronegativity[edit | edit source]
The electronegativity of the atoms involved in the acid molecule can affect its strength. Generally, the more electronegative the atom bonded to the hydrogen, the stronger the acid. This is because electronegative atoms can stabilize the negative charge of the conjugate base more effectively.
Bond Strength[edit | edit source]
The strength of the bond between the hydrogen and the rest of the acid molecule also plays a role. Weaker bonds are more easily broken, making the acid stronger. For example, the bond strength in hydroiodic acid (HI) is weaker than in hydrochloric acid (HCl), making HI a stronger acid.
Inductive Effect[edit | edit source]
The inductive effect refers to the electron-withdrawing or electron-donating effects of substituents attached to the acid molecule. Electron-withdrawing groups, such as halogens, can increase acid strength by stabilizing the negative charge on the conjugate base. For example, chlorinated acetic acids are stronger than acetic acid due to the electron-withdrawing effect of chlorine.
Resonance[edit | edit source]
Resonance stabilization of the conjugate base can also enhance acid strength. If the negative charge on the conjugate base can be delocalized over several atoms, the acid is generally stronger. This is seen in carboxylic acids, where the conjugate base is stabilized by resonance.
Examples of Acid Strength[edit | edit source]
The strength of acids can be illustrated by comparing the dissociation of different acids:
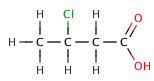
Butanoic Acids[edit | edit source]
Butanoic acid (C₃H₇COOH) is a weak acid, as it only partially dissociates in water. Its strength can be compared to its chlorinated derivatives, such as 2-chlorobutanoic acid, 3-chlorobutanoic acid, and 4-chlorobutanoic acid, which are stronger due to the inductive effect of the chlorine atoms.
Related Pages[edit | edit source]
Search WikiMD
Ad.Tired of being Overweight? Try W8MD's physician weight loss program.
Semaglutide (Ozempic / Wegovy and Tirzepatide (Mounjaro / Zepbound) available.
Advertise on WikiMD
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Translate this page: - East Asian
中文,
日本,
한국어,
South Asian
हिन्दी,
தமிழ்,
తెలుగు,
Urdu,
ಕನ್ನಡ,
Southeast Asian
Indonesian,
Vietnamese,
Thai,
မြန်မာဘာသာ,
বাংলা
European
español,
Deutsch,
français,
Greek,
português do Brasil,
polski,
română,
русский,
Nederlands,
norsk,
svenska,
suomi,
Italian
Middle Eastern & African
عربى,
Turkish,
Persian,
Hebrew,
Afrikaans,
isiZulu,
Kiswahili,
Other
Bulgarian,
Hungarian,
Czech,
Swedish,
മലയാളം,
मराठी,
ਪੰਜਾਬੀ,
ગુજરાતી,
Portuguese,
Ukrainian
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Contributors: Prab R. Tumpati, MD