Zeeman effect
The Zeeman Effect refers to the phenomenon discovered by the Dutch physicist Pieter Zeeman in 1896, which involves the splitting of a spectral line into several components in the presence of a static magnetic field. This effect is a key piece of evidence for the quantum mechanical nature of atoms and electrons, and it has profound implications in the fields of physics and astronomy.
Overview[edit | edit source]
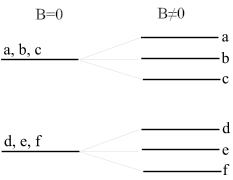
When light is emitted or absorbed by atoms in a magnetic field, the energy levels of the electrons in the atoms are split. This splitting occurs because the magnetic field affects the motion of the electrons, and thus, their energy states. The Zeeman Effect can be observed as a splitting of the spectral lines, which are the lines that appear in a spectroscope when light is dispersed. These lines represent the energies at which electrons can absorb or emit light. The effect can be classified into the Normal Zeeman Effect and the Anomalous Zeeman Effect, depending on the nature of the splitting.
Normal Zeeman Effect[edit | edit source]
The Normal Zeeman Effect is observed when the splitting of the spectral line is into three components: one at the original wavelength and two others at slightly shifted wavelengths. This occurs in the presence of a relatively weak magnetic field and is explained by the Lorentz force acting on the moving electrons. The Normal Zeeman Effect can be fully explained using the classical theory of electromagnetism.
Anomalous Zeeman Effect[edit | edit source]
The Anomalous Zeeman Effect refers to the splitting of spectral lines into more than three components. This effect is observed in stronger magnetic fields and cannot be explained by classical physics alone. The explanation for the Anomalous Zeeman Effect comes from quantum mechanics, specifically the interaction between the magnetic field and the intrinsic spin of the electron, in addition to its orbital angular momentum.
Applications[edit | edit source]
The Zeeman Effect has numerous applications in both theoretical and applied physics. It is used in astronomy to measure the magnetic fields of stars and galaxies, as well as in atomic clocks, which are highly precise timekeeping devices. In laboratory settings, the Zeeman Effect is utilized in techniques such as laser cooling and magnetic resonance imaging (MRI).
Historical Significance[edit | edit source]
The discovery of the Zeeman Effect was pivotal in the development of quantum mechanics. It provided one of the first pieces of evidence that light and matter interact in a quantized manner. For his discovery, Pieter Zeeman was awarded the Nobel Prize in Physics in 1902, shared with Hendrik Lorentz, who provided a theoretical explanation for the effect.
Search WikiMD
Ad.Tired of being Overweight? Try W8MD's physician weight loss program.
Semaglutide (Ozempic / Wegovy and Tirzepatide (Mounjaro / Zepbound) available.
Advertise on WikiMD
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Translate this page: - East Asian
中文,
日本,
한국어,
South Asian
हिन्दी,
தமிழ்,
తెలుగు,
Urdu,
ಕನ್ನಡ,
Southeast Asian
Indonesian,
Vietnamese,
Thai,
မြန်မာဘာသာ,
বাংলা
European
español,
Deutsch,
français,
Greek,
português do Brasil,
polski,
română,
русский,
Nederlands,
norsk,
svenska,
suomi,
Italian
Middle Eastern & African
عربى,
Turkish,
Persian,
Hebrew,
Afrikaans,
isiZulu,
Kiswahili,
Other
Bulgarian,
Hungarian,
Czech,
Swedish,
മലയാളം,
मराठी,
ਪੰਜਾਬੀ,
ગુજરાતી,
Portuguese,
Ukrainian
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Contributors: Prab R. Tumpati, MD