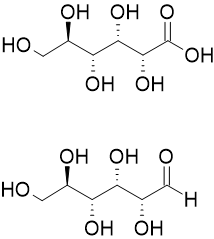
Aldonic acid
Aldonic Acid
Aldonic acids are a type of sugar acid derived from the oxidation of the aldose group of a sugar molecule. They are characterized by the presence of a carboxylic acid group on the terminal carbon of the sugar chain. Aldonic acids play a significant role in various biological processes and have applications in food science, pharmaceuticals, and other industries.
Structure and Properties[edit | edit source]
Aldonic acids are typically formed by the oxidation of the primary alcohol group of an aldose sugar to a carboxylic acid group. This oxidation reaction can be enzymatically catalyzed or chemically induced. The resulting aldonic acid retains the same carbon backbone as the original aldose sugar but with an additional carboxylic acid functional group. The presence of the carboxylic acid group imparts unique properties to aldonic acids, including increased water solubility and acidity compared to their parent aldose sugars. These properties make aldonic acids useful as chelating agents, preservatives, and flavor enhancers in various applications.
Biological Significance[edit | edit source]
In biological systems, aldonic acids can be intermediates in the metabolism of sugars. Enzymatic oxidation of aldose sugars by organisms such as bacteria and fungi can lead to the production of aldonic acids as part of their metabolic pathways. These acids can serve as a source of energy or as precursors for the synthesis of other biomolecules. Aldonic acids have also been implicated in various physiological processes, including cell signaling and immune responses. Research on the role of aldonic acids in health and disease is ongoing, with potential implications for understanding metabolic disorders and developing new therapeutic strategies.
Applications[edit | edit source]
The unique properties of aldonic acids make them valuable in a range of applications. In the food industry, aldonic acids are used as natural preservatives and acidulants in beverages, dairy products, and baked goods. Their ability to chelate metal ions also makes them useful in food fortification and as antioxidants. In pharmaceuticals, aldonic acids have potential applications in drug delivery systems and as components of novel therapeutics. Their water solubility and biocompatibility make them attractive candidates for various pharmaceutical formulations. In addition to their roles in food and pharmaceuticals, aldonic acids are being explored for applications in biotechnology, materials science, and environmental remediation. Ongoing research aims to harness the unique properties of aldonic acids for diverse industrial and scientific purposes.
See Also[edit | edit source]
Transform your life with W8MD's budget GLP1 injections from $125 and up biweekly
W8MD offers a medical weight loss program NYC and a clinic to lose weight in Philadelphia. Our W8MD's physician supervised medical weight loss centers in NYC provides expert medical guidance, and offers telemedicine options for convenience.
Why choose W8MD?
- Comprehensive care with FDA-approved weight loss medications including:
- loss injections in NYC both generic and brand names:
- weight loss medications including Phentermine, Qsymia, Contrave, Diethylpropion etc.
- Accept most insurances for visits or discounted self pay cost.
- Generic weight loss injections starting from just $125.00 for the starting dose
- In person weight loss NYC and telemedicine medical weight loss options in New York city available
Book Your Appointment
Start your NYC weight loss journey today at our NYC medical weight loss, and Philadelphia and visit Philadelphia medical weight loss Call (718)946-5500 for NY and 215 676 2334 for PA
Search WikiMD
Ad.Tired of being Overweight? Try W8MD's NYC physician weight loss.
Semaglutide (Ozempic / Wegovy and Tirzepatide (Mounjaro / Zepbound) available. Call 718 946 5500.
Advertise on WikiMD
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Translate this page: - East Asian
中文,
日本,
한국어,
South Asian
हिन्दी,
தமிழ்,
తెలుగు,
Urdu,
ಕನ್ನಡ,
Southeast Asian
Indonesian,
Vietnamese,
Thai,
မြန်မာဘာသာ,
বাংলা
European
español,
Deutsch,
français,
Greek,
português do Brasil,
polski,
română,
русский,
Nederlands,
norsk,
svenska,
suomi,
Italian
Middle Eastern & African
عربى,
Turkish,
Persian,
Hebrew,
Afrikaans,
isiZulu,
Kiswahili,
Other
Bulgarian,
Hungarian,
Czech,
Swedish,
മലയാളം,
मराठी,
ਪੰਜਾਬੀ,
ગુજરાતી,
Portuguese,
Ukrainian
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Contributors: Prab R. Tumpati, MD