Grotthuss mechanism
Grotthuss Mechanism[edit | edit source]
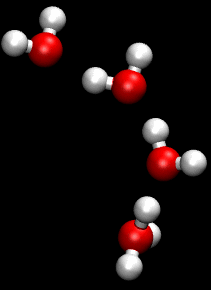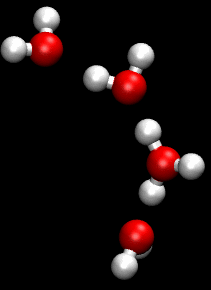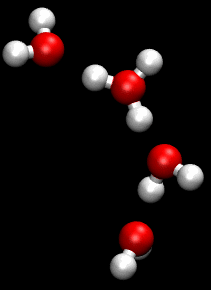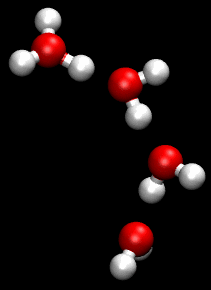
The Grotthuss mechanism, also known as the proton hopping mechanism, is a model that describes the movement of protons (H_ ions) through a network of hydrogen-bonded water molecules. This mechanism is fundamental to understanding the conductivity of protons in aqueous solutions and is named after Theodor Grotthuss, who proposed the concept in 1806.
Mechanism Description[edit | edit source]
The Grotthuss mechanism involves the transfer of protons between water molecules, facilitated by the formation and breaking of hydrogen bonds. In this process, a proton is transferred from one water molecule to another, creating a chain reaction that allows for rapid proton mobility. This is distinct from the movement of other ions, which typically involves the physical movement of the ion itself through the solution.
Proton Transfer[edit | edit source]
In the Grotthuss mechanism, a proton initially associated with a hydronium ion (H_O_) is transferred to a neighboring water molecule, converting it into a new hydronium ion. This process continues along a chain of water molecules, effectively "hopping" the proton through the network. The overall effect is a rapid movement of charge without the need for the physical displacement of the hydronium ion itself.
Zundel and Eigen Complexes[edit | edit source]
Two important intermediates in the Grotthuss mechanism are the Zundel complex and the Eigen complex. The Zundel complex (H_O__) involves a proton shared equally between two water molecules, while the Eigen complex (H_O__) consists of a central hydronium ion surrounded by three water molecules. These complexes play a crucial role in facilitating proton transfer by stabilizing the transition states involved in the hopping process.
Importance in Chemistry[edit | edit source]
The Grotthuss mechanism is essential for understanding the high proton conductivity observed in water and other hydrogen-bonded networks. It is a key concept in fields such as electrochemistry, biochemistry, and materials science, where proton transport is a critical factor. The mechanism also has implications for the design of fuel cells and other technologies that rely on efficient proton conduction.
Related Pages[edit | edit source]
Search WikiMD
Ad.Tired of being Overweight? Try W8MD's physician weight loss program.
Semaglutide (Ozempic / Wegovy and Tirzepatide (Mounjaro / Zepbound) available.
Advertise on WikiMD
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Translate this page: - East Asian
中文,
日本,
한국어,
South Asian
हिन्दी,
தமிழ்,
తెలుగు,
Urdu,
ಕನ್ನಡ,
Southeast Asian
Indonesian,
Vietnamese,
Thai,
မြန်မာဘာသာ,
বাংলা
European
español,
Deutsch,
français,
Greek,
português do Brasil,
polski,
română,
русский,
Nederlands,
norsk,
svenska,
suomi,
Italian
Middle Eastern & African
عربى,
Turkish,
Persian,
Hebrew,
Afrikaans,
isiZulu,
Kiswahili,
Other
Bulgarian,
Hungarian,
Czech,
Swedish,
മലയാളം,
मराठी,
ਪੰਜਾਬੀ,
ગુજરાતી,
Portuguese,
Ukrainian
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Contributors: Prab R. Tumpati, MD