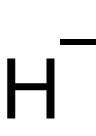Hydride ion
The hydride ion is a simple anion with the chemical formula H−. It consists of a hydrogen atom that has gained an extra electron, resulting in a negative charge. This ion is a key component in various branches of chemistry, including inorganic chemistry, organic chemistry, and biochemistry. Hydride ions play a crucial role in many chemical reactions, including reductions and acid-base reactions.
Properties[edit | edit source]
The hydride ion is characterized by its high reactivity and strong basicity. It is considered a strong base because it readily donates its extra electron to form a covalent bond with other atoms or molecules, effectively reducing them. The ion's reactivity is attributed to the high charge density on the small hydrogen nucleus, which makes it eager to bond with other atoms or molecules that can accept an electron.
Formation[edit | edit source]
Hydride ions can be formed in several ways, including the direct reaction of hydrogen gas (H2) with metals, especially those from the alkali metals and alkaline earth metals groups, under specific conditions. This process typically involves the transfer of an electron from the metal to the hydrogen, resulting in the formation of a metal hydride and the release of a hydride ion.
Role in Chemical Reactions[edit | edit source]
In organic chemistry, hydride ions are often used as reducing agents, capable of donating electrons to various substrates, leading to the reduction of ketones, aldehydes, and other carbonyl compounds to alcohols. In inorganic chemistry, hydride ions can react with metal cations to form metal hydrides, which are important in various industrial applications, including energy storage and catalysis.
Biological Significance[edit | edit source]
In biochemistry, hydride ions are involved in several essential biochemical reactions, including those occurring in the electron transport chain during cellular respiration. They play a critical role in the conversion of NAD+ to NADH, a key step in the process of ATP production, which is the primary energy currency of the cell.
Safety and Handling[edit | edit source]
Due to their high reactivity, hydride ions and compounds containing them should be handled with care. They can react violently with water and other proton donors to release hydrogen gas, which is flammable and potentially explosive.
Search WikiMD
Ad.Tired of being Overweight? Try W8MD's physician weight loss program.
Semaglutide (Ozempic / Wegovy and Tirzepatide (Mounjaro / Zepbound) available.
Advertise on WikiMD
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Translate this page: - East Asian
中文,
日本,
한국어,
South Asian
हिन्दी,
தமிழ்,
తెలుగు,
Urdu,
ಕನ್ನಡ,
Southeast Asian
Indonesian,
Vietnamese,
Thai,
မြန်မာဘာသာ,
বাংলা
European
español,
Deutsch,
français,
Greek,
português do Brasil,
polski,
română,
русский,
Nederlands,
norsk,
svenska,
suomi,
Italian
Middle Eastern & African
عربى,
Turkish,
Persian,
Hebrew,
Afrikaans,
isiZulu,
Kiswahili,
Other
Bulgarian,
Hungarian,
Czech,
Swedish,
മലയാളം,
मराठी,
ਪੰਜਾਬੀ,
ગુજરાતી,
Portuguese,
Ukrainian
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Contributors: Prab R. Tumpati, MD