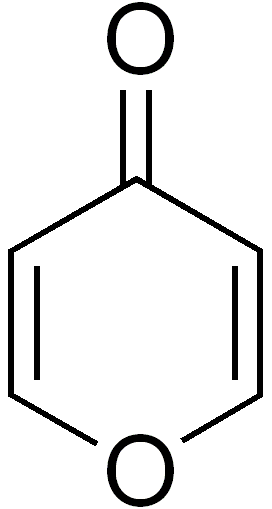
Pyrone
Pyrone is an organic compound with the molecular formula C₅H₄O₂. It is a heterocyclic compound that contains a six-membered ring with two double bonds and an oxygen atom. Pyrones are divided into two isomers: α-pyrone and γ-pyrone, which differ in the position of the carbonyl group.
Structure and Isomers[edit | edit source]
Pyrones are characterized by a six-membered ring structure containing one oxygen atom and two double bonds. The two main isomers of pyrone are:
- α-Pyrone (2-pyrone): The carbonyl group is located at the second position of the ring.
- γ-Pyrone (4-pyrone): The carbonyl group is located at the fourth position of the ring.
Synthesis[edit | edit source]
Pyrones can be synthesized through various chemical reactions. One common method involves the cyclization of β-keto esters in the presence of an acid catalyst. Another method includes the Perkin reaction, which involves the condensation of an aromatic aldehyde with an anhydride.
Reactivity[edit | edit source]
Pyrones exhibit unique reactivity due to the presence of the carbonyl group and the conjugated double bonds. They can undergo various chemical reactions, including:
- Diels-Alder reaction: Pyrones can act as dienes in Diels-Alder reactions, forming cyclohexene derivatives.
- Nucleophilic addition: The carbonyl group in pyrones can undergo nucleophilic addition reactions.
Applications[edit | edit source]
Pyrones are important in various fields, including:
- Pharmaceuticals: Some pyrone derivatives have biological activity and are used in the development of drugs.
- Flavors and fragrances: Pyrones contribute to the aroma and flavor of certain foods and are used in the fragrance industry.
Examples of Pyrones[edit | edit source]
Several naturally occurring and synthetic pyrones are of interest, including:
- Coumarin: A naturally occurring α-pyrone found in many plants.
- Kojic acid: A γ-pyrone derivative used in cosmetics and food products.
See Also[edit | edit source]
References[edit | edit source]
External Links[edit | edit source]
Search WikiMD
Ad.Tired of being Overweight? Try W8MD's NYC physician weight loss.
Semaglutide (Ozempic / Wegovy and Tirzepatide (Mounjaro / Zepbound) available. Call 718 946 5500.
Advertise on WikiMD
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Translate this page: - East Asian
中文,
日本,
한국어,
South Asian
हिन्दी,
தமிழ்,
తెలుగు,
Urdu,
ಕನ್ನಡ,
Southeast Asian
Indonesian,
Vietnamese,
Thai,
မြန်မာဘာသာ,
বাংলা
European
español,
Deutsch,
français,
Greek,
português do Brasil,
polski,
română,
русский,
Nederlands,
norsk,
svenska,
suomi,
Italian
Middle Eastern & African
عربى,
Turkish,
Persian,
Hebrew,
Afrikaans,
isiZulu,
Kiswahili,
Other
Bulgarian,
Hungarian,
Czech,
Swedish,
മലയാളം,
मराठी,
ਪੰਜਾਬੀ,
ગુજરાતી,
Portuguese,
Ukrainian
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Contributors: Prab R. Tumpati, MD