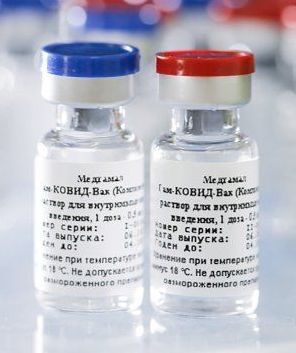
Sputnik V COVID-19 vaccine
Sputnik V COVID-19 vaccine
The Sputnik V COVID-19 vaccine (Russian: Спутник V) is a COVID-19 vaccine developed by the Gamaleya Research Institute of Epidemiology and Microbiology in Russia. It is an adenovirus viral vector vaccine, which means it uses a modified virus to deliver genetic material from the SARS-CoV-2 virus into human cells to stimulate an immune response.
Development and Approval[edit | edit source]
The development of Sputnik V began in early 2020, shortly after the World Health Organization declared the COVID-19 outbreak a pandemic. The vaccine was named after the Sputnik 1 satellite, the first artificial satellite launched by the Soviet Union in 1957. The vaccine was registered by the Russian Ministry of Health on August 11, 2020, making it the first COVID-19 vaccine to be approved for use in any country.
Mechanism of Action[edit | edit source]
Sputnik V uses two different adenoviruses (rAd26 and rAd5) for the first and second doses, respectively. This heterologous prime-boost vaccination strategy is designed to enhance the immune response. The adenoviruses are engineered to carry the gene for the SARS-CoV-2 spike protein, which prompts the body to produce an immune response against the virus.
Efficacy and Safety[edit | edit source]
Clinical trials have shown that Sputnik V has an efficacy rate of approximately 91.6%. The vaccine has been shown to be effective in preventing symptomatic COVID-19 and severe cases of the disease. Common side effects include mild flu-like symptoms, pain at the injection site, and fatigue.
Distribution and Use[edit | edit source]
Sputnik V has been approved for emergency use in over 70 countries worldwide. It is distributed in two doses, administered 21 days apart. The vaccine can be stored at temperatures between 2-8 degrees Celsius, making it easier to distribute compared to some other COVID-19 vaccines that require ultra-cold storage.
Controversies and Criticisms[edit | edit source]
The early approval of Sputnik V raised concerns among some scientists and public health experts due to the lack of published data from large-scale clinical trials at the time of its registration. However, subsequent peer-reviewed studies have supported its efficacy and safety.
Related Pages[edit | edit source]
- COVID-19 pandemic
- COVID-19 vaccine
- Gamaleya Research Institute of Epidemiology and Microbiology
- Adenovirus
- SARS-CoV-2
- World Health Organization
See Also[edit | edit source]
- Pfizer–BioNTech COVID-19 vaccine
- Moderna COVID-19 vaccine
- AstraZeneca COVID-19 vaccine
- Johnson & Johnson COVID-19 vaccine
References[edit | edit source]
External Links[edit | edit source]
Lua error in package.lua at line 80: module 'strict' not found.
Search WikiMD
Ad.Tired of being Overweight? Try W8MD's NYC physician weight loss.
Semaglutide (Ozempic / Wegovy and Tirzepatide (Mounjaro / Zepbound) available. Call 718 946 5500.
Advertise on WikiMD
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Translate this page: - East Asian
中文,
日本,
한국어,
South Asian
हिन्दी,
தமிழ்,
తెలుగు,
Urdu,
ಕನ್ನಡ,
Southeast Asian
Indonesian,
Vietnamese,
Thai,
မြန်မာဘာသာ,
বাংলা
European
español,
Deutsch,
français,
Greek,
português do Brasil,
polski,
română,
русский,
Nederlands,
norsk,
svenska,
suomi,
Italian
Middle Eastern & African
عربى,
Turkish,
Persian,
Hebrew,
Afrikaans,
isiZulu,
Kiswahili,
Other
Bulgarian,
Hungarian,
Czech,
Swedish,
മലയാളം,
मराठी,
ਪੰਜਾਬੀ,
ગુજરાતી,
Portuguese,
Ukrainian
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Contributors: Prab R. Tumpati, MD