Transition state theory
Transition State Theory (TST), also known as Activated-Complex Theory, is a concept in chemical kinetics and physical chemistry that describes how chemical reactions occur and how reaction rates are determined. It provides a detailed account of the transition state - a high-energy, unstable arrangement of atoms that exists momentarily at the peak of the reaction coordinate. Understanding TST is crucial for the development of new chemical processes, including the design of catalysts that can lower the activation energy required for a reaction.
Overview[edit | edit source]
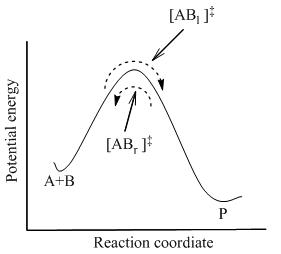
At the heart of transition state theory is the concept of the activation energy (Ea), the energy barrier that must be overcome for reactants to transform into products. According to TST, molecules form a transition state or activated complex during the reaction. This complex can either revert to reactants or proceed to form products. The theory assumes that the transition state is in a quasi-equilibrium with the reactants, allowing the use of thermodynamics to describe the system.
Theoretical Background[edit | edit source]
Transition state theory is grounded in the principles of statistical mechanics. It uses the concept of a potential energy surface (PES) to describe the energy changes that occur during a reaction. The PES maps the energy of the system as a function of the positions of all the atoms involved in the reaction. The path of lowest energy that connects the reactants to the products is known as the reaction coordinate, and the highest point on this path is the transition state.
Key Equations[edit | edit source]
The rate of a chemical reaction according to TST is given by the Eyring equation, derived from the Arrhenius equation:
\[ k = \frac{k_B T}{h} e^{-\frac{\Delta G^\ddagger}{RT}} \]
where:
- \(k\) is the reaction rate constant,
- \(k_B\) is the Boltzmann constant,
- \(T\) is the temperature,
- \(h\) is Planck's constant,
- \(\Delta G^\ddagger\) is the Gibbs free energy of activation,
- \(R\) is the gas constant.
This equation highlights the dependence of the reaction rate on the temperature and the Gibbs free energy of the transition state.
Applications[edit | edit source]
Transition state theory has wide-ranging applications in chemistry, biochemistry, and materials science. It is used to understand and predict the rates of chemical reactions, design chemical processes, and develop new materials and drugs. In enzyme catalysis, TST helps explain how enzymes reduce the activation energy of biochemical reactions, thereby increasing reaction rates.
Limitations[edit | edit source]
While TST provides a robust framework for understanding chemical kinetics, it has limitations. It assumes a well-defined transition state, which may not exist in some complex reactions. Additionally, TST does not account for the role of solvent effects or non-equilibrium dynamics that can influence reaction rates.
Conclusion[edit | edit source]
Transition State Theory remains a fundamental concept in the study of chemical reactions. Its development has significantly advanced our understanding of reaction mechanisms and kinetics, despite its limitations. Ongoing research in the field seeks to extend and refine TST to better understand complex chemical and biochemical systems.
Search WikiMD
Ad.Tired of being Overweight? Try W8MD's physician weight loss program.
Semaglutide (Ozempic / Wegovy and Tirzepatide (Mounjaro / Zepbound) available.
Advertise on WikiMD
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Translate this page: - East Asian
中文,
日本,
한국어,
South Asian
हिन्दी,
தமிழ்,
తెలుగు,
Urdu,
ಕನ್ನಡ,
Southeast Asian
Indonesian,
Vietnamese,
Thai,
မြန်မာဘာသာ,
বাংলা
European
español,
Deutsch,
français,
Greek,
português do Brasil,
polski,
română,
русский,
Nederlands,
norsk,
svenska,
suomi,
Italian
Middle Eastern & African
عربى,
Turkish,
Persian,
Hebrew,
Afrikaans,
isiZulu,
Kiswahili,
Other
Bulgarian,
Hungarian,
Czech,
Swedish,
മലയാളം,
मराठी,
ਪੰਜਾਬੀ,
ગુજરાતી,
Portuguese,
Ukrainian
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Contributors: Prab R. Tumpati, MD