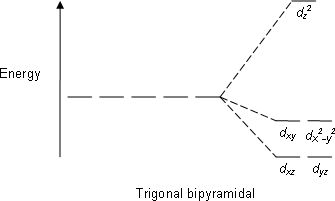
Trigonal bipyramidal
The trigonal bipyramidal molecular geometry is a type of molecular shape that occurs when a central atom is surrounded by five atoms in a molecule. This shape is characterized by three atoms forming an equatorial plane around the central atom, with the remaining two atoms (the axial atoms) positioned above and below this plane, creating a shape reminiscent of two pyramids joined at their bases. The trigonal bipyramidal geometry is crucial in the field of chemistry, particularly in inorganic chemistry and molecular geometry.
The angles between the equatorial atoms are 120 degrees, while the angles between an equatorial and an axial atom are 90 degrees. This geometry is often described using the VSEPR theory (Valence Shell Electron Pair Repulsion theory), which predicts the shape of molecules based on the repulsion between electron pairs in the valence shell of the central atom.
One of the key aspects of trigonal bipyramidal geometry is the concept of ligand exchange mechanisms, particularly the Berry pseudorotation, which explains the interconversion between different isomers of a molecule by the movement of ligands between axial and equatorial positions.
Phosphorus pentachloride (PCl5) is a classic example of a compound exhibiting trigonal bipyramidal geometry, with phosphorus as the central atom surrounded by five chlorine atoms. Other examples include iron pentacarbonyl (Fe(CO)5) and various organophosphorus compounds.
The understanding of trigonal bipyramidal geometry is essential for predicting the reactivity, polarity, and physical properties of molecules. It also plays a significant role in the synthesis and design of new compounds in organic chemistry and coordination chemistry.
Search WikiMD
Ad.Tired of being Overweight? Try W8MD's physician weight loss program.
Semaglutide (Ozempic / Wegovy and Tirzepatide (Mounjaro / Zepbound) available.
Advertise on WikiMD
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Translate this page: - East Asian
中文,
日本,
한국어,
South Asian
हिन्दी,
தமிழ்,
తెలుగు,
Urdu,
ಕನ್ನಡ,
Southeast Asian
Indonesian,
Vietnamese,
Thai,
မြန်မာဘာသာ,
বাংলা
European
español,
Deutsch,
français,
Greek,
português do Brasil,
polski,
română,
русский,
Nederlands,
norsk,
svenska,
suomi,
Italian
Middle Eastern & African
عربى,
Turkish,
Persian,
Hebrew,
Afrikaans,
isiZulu,
Kiswahili,
Other
Bulgarian,
Hungarian,
Czech,
Swedish,
മലയാളം,
मराठी,
ਪੰਜਾਬੀ,
ગુજરાતી,
Portuguese,
Ukrainian
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates Wikipedia, licensed under CC BY SA or similar.
Contributors: Prab R. Tumpati, MD