Gadoterate meglumine
(Redirected from Dotarem)
What is Gadoterate meglumine?[edit | edit source]
- Gadoterate meglumine (DOTAREM) is a gadolinium-based contrast agent used with a magnetic resonance imaging (MRI) scanner.
What are the uses of this medicine?[edit | edit source]
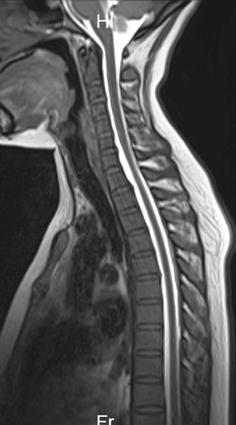
- DOTAREM is a gadolinium-based contrast agent indicated for intravenous use with magnetic resonance imaging (MRI) in brain (intracranial), spine and associated tissues in adult and pediatric patients (including term neonates) to detect and visualize areas with disruption of the blood brain barrier (BBB) and/or abnormal vascularity.
How does this medicine work?[edit | edit source]
- Gadoterate is a paramagnetic molecule that develops a magnetic moment when placed in a magnetic field.
- The magnetic moment enhances the relaxation rates of water protons in its vicinity, leading to an increase in signal intensity (brightness) of tissues.
- When placed in a magnetic field, gadoterate shortens the T1 and T2 relaxation times in target tissues.
- At recommended doses, the effect is observed with greatest sensitivity in the T1-weighted sequences.
Who Should Not Use this medicine ?[edit | edit source]
This medicine cannot be used in patients who:
- have had a severe allergic reaction to DOTAREM
What drug interactions can this medicine cause?[edit | edit source]
- Specific drug interaction studies with DOTAREM have not been conducted.
Is this medicine FDA approved?[edit | edit source]
- FDA approved this drug in the year of 2013.
How should this medicine be used?[edit | edit source]
Recommended Dosage:
- The recommended dose of DOTAREM is 0.2 mL/kg (0.1 mmol/kg) body weight administered as an intravenous bolus injection at a flow rate of approximately 2 mL/second for adults and 1 - 2 mL/second for pediatric patients (including term neonates).
- The dose is delivered by manual or power injection.
Administration
- Visually inspect DOTAREM for particulate matter prior to administration. Do not use the solution if particulate matter is present or if the container appears damaged. DOTAREM should be a clear, colorless to yellow solution.
- Do not mix with other drugs or parenteral nutrition.
- Discard any unused portions of the drug.
- Holding the syringe barrel firmly, screw the threaded tip of the plunger rod clockwise into the cartridge plunger.
- Holding the syringe vertically so the rubber cap is pointed upward, aseptically remove the rubber cap from the tip of the syringe and attach either a sterile, disposable needle or compatible needleless luer lock tubing set using a push-twist action. At this point, the tubing set is not attached to a patient’s intravenous connection.
- If using a needleless luer lock tubing set, check the connection between the syringe and the tubing as the fluid flows. Ensure that the connection is successful before administration of DOTAREM Injection.
- If using a needle, hold the syringe vertically and push plunger forward until all of the air is evacuated and fluid either appears at the tip of the needle or the tubing is filled. Following the usual venous blood aspiration procedure, complete the DOTAREM injection.
- To ensure complete delivery of the contrast medium, the injection may be followed by a normal saline flush.
- Properly dispose of the syringe and any other materials used.
What are the dosage forms and brand names of this medicine?[edit | edit source]
This medicine is available in fallowing doasage form:
- As DOTAREM Injection 0.5 mmol/mL contains 376.9 mg/mL of gadoterate meglumine and is available in vials and pre-filled syringes.
This medicine is available in fallowing brand namesː
- DOTAREM
What side effects can this medication cause?[edit | edit source]
The most common side effects of this medicine include:
- nausea
- headache
- pain
- cold feeling at the injection site
- rash
What special precautions should I follow?[edit | edit source]
- Nephrogenic Systemic Fibrosis has occurred in patients with impaired elimination of GBCAs. Higher than recommended dosing or repeat dosing appear to increase the risk.
- Anaphylactoid/anaphylactic reactions with cardiovascular, respiratory or cutaneous manifestations, ranging from mild to severe, including death, have uncommonly occurred. Monitor patients closely for need of emergency cardiorespiratory support.
- Gadolinium is retained for months or years in brain, bone, and other organs.
- In patients with chronically reduced renal function, acute kidney injury requiring dialysis has occurred with the use of GBCAs. Consider follow-up renal function assessments for patients with a history of renal dysfunction.
What to do in case of emergency/overdose?[edit | edit source]
- Adverse reactions to overdosage with DOTAREM have not been reported.
Management of overdosage:
- Gadoterate meglumine can be removed from the body by hemodialysis.
Can this medicine be used in pregnancy?[edit | edit source]
- GBCAs cross the human placenta and result in fetal exposure and gadolinium retention. The human data on the association between GBCAs and adverse fetal outcomes.
- Because of the potential risks of gadolinium to the fetus, use DOTAREM only if imaging is essential during pregnancy and cannot be delayed.
Can this medicine be used in children?[edit | edit source]
- The safety and efficacy of DOTAREM at a single dose of 0.1 mmol/kg have been established in pediatric patients from birth.
- The safety of DOTAREM has not been established in preterm neonates.
What are the active and inactive ingredients in this medicine?[edit | edit source]
- Active ingredient: gadoterate meglumine
- Inactive ingredients: DOTA, water for injection
Who manufactures and distributes this medicine?[edit | edit source]
- Manufactured by: Catalent (glass pre-filled syringes), Liebel-Flarsheim Company LLC (plastic pre-filled syringes and vials) and Recipharm (vials) for Guerbet
What should I know about storage and disposal of this medication?[edit | edit source]
- Store at 25°C (77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) .
- Pre-filled syringes must not be frozen. Frozen syringes should be discarded.
- Should solidification occur in the vial because of exposure to the cold, bring DOTAREM to room temperature before use.
- If allowed to stand at room temperature for a minimum of 90 minutes, DOTAREM should return to a clear, colorless to yellow solution.
- Before use, examine the product to assure that all solids are dissolved and that the container and closure have not been damaged.
- Discard the vial if solids persist.
| Contrast media (V08) | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Search WikiMD
Ad.Tired of being Overweight? Try W8MD's physician weight loss program.
Semaglutide (Ozempic / Wegovy and Tirzepatide (Mounjaro / Zepbound) available.
Advertise on WikiMD
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Translate this page: - East Asian
中文,
日本,
한국어,
South Asian
हिन्दी,
தமிழ்,
తెలుగు,
Urdu,
ಕನ್ನಡ,
Southeast Asian
Indonesian,
Vietnamese,
Thai,
မြန်မာဘာသာ,
বাংলা
European
español,
Deutsch,
français,
Greek,
português do Brasil,
polski,
română,
русский,
Nederlands,
norsk,
svenska,
suomi,
Italian
Middle Eastern & African
عربى,
Turkish,
Persian,
Hebrew,
Afrikaans,
isiZulu,
Kiswahili,
Other
Bulgarian,
Hungarian,
Czech,
Swedish,
മലയാളം,
मराठी,
ਪੰਜਾਬੀ,
ગુજરાતી,
Portuguese,
Ukrainian
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Contributors: Deepika vegiraju