Phosphoglycerate mutase
(Redirected from Phosphoglyceromutase)
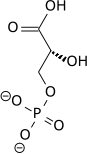
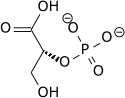
Phosphoglycerate mutase (PGAM or PGM) is an enzyme that plays a crucial role in the process of glycolysis and gluconeogenesis. This enzyme is involved in the step that interconverts 3-phosphoglycerate (3-PG) and 2-phosphoglycerate (2-PG) during cellular respiration. Phosphoglycerate mutase exists in different isoforms, which are found in various types of tissues in the body, indicating its essential role in energy metabolism across different cell types.
Function[edit | edit source]
The primary function of phosphoglycerate mutase is to catalyze the transfer of a phosphate group from the 3-position to the 2-position in glycerate phosphate. This reaction is reversible and plays a vital role in both the glycolytic pathway, where glucose is broken down to produce energy, and gluconeogenesis, the process of glucose synthesis. The enzyme's action facilitates the metabolic pathway's progression towards the production of pyruvate in glycolysis or towards the generation of glucose in gluconeogenesis.
Structure[edit | edit source]
Phosphoglycerate mutase exists mainly in two forms: 1) as a dimeric enzyme which is primarily found in muscle tissues (PGAM1), and 2) as a monomeric form which is more common in non-muscle tissues. The structure of PGAM includes an active site that binds to the substrate (3-phosphoglycerate or 2-phosphoglycerate) and a catalytic site that facilitates the transfer of the phosphate group. The enzyme's activity is dependent on the presence of 2,3-bisphosphoglycerate (2,3-BPG) as a cofactor in the glycolytic pathway.
Isoforms[edit | edit source]
There are several isoforms of phosphoglycerate mutase, each encoded by different genes and expressed in various tissues. The most well-known isoforms are:
- PGAM1, which is predominantly found in muscle tissue and is involved in rapid energy production during muscle contraction.
- PGAM2, which is expressed in other tissues and plays a role in the regular energy metabolism of cells.
Clinical Significance[edit | edit source]
Alterations in the activity or expression of phosphoglycerate mutase can have significant implications for human health. Deficiencies in PGAM2, for example, can lead to a metabolic disorder known as glycogen storage disease type X, characterized by muscle cramps and exercise intolerance due to impaired glycolytic flux in muscle cells. Furthermore, changes in PGAM1 expression have been observed in various types of cancer, suggesting that the enzyme could play a role in cancer metabolism and might serve as a potential target for therapeutic intervention.
See Also[edit | edit source]
This article is a biochemistry stub. You can help WikiMD by expanding it!
Search WikiMD
Ad.Tired of being Overweight? Try W8MD's physician weight loss program.
Semaglutide (Ozempic / Wegovy and Tirzepatide (Mounjaro / Zepbound) available.
Advertise on WikiMD
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Translate this page: - East Asian
中文,
日本,
한국어,
South Asian
हिन्दी,
தமிழ்,
తెలుగు,
Urdu,
ಕನ್ನಡ,
Southeast Asian
Indonesian,
Vietnamese,
Thai,
မြန်မာဘာသာ,
বাংলা
European
español,
Deutsch,
français,
Greek,
português do Brasil,
polski,
română,
русский,
Nederlands,
norsk,
svenska,
suomi,
Italian
Middle Eastern & African
عربى,
Turkish,
Persian,
Hebrew,
Afrikaans,
isiZulu,
Kiswahili,
Other
Bulgarian,
Hungarian,
Czech,
Swedish,
മലയാളം,
मराठी,
ਪੰਜਾਬੀ,
ગુજરાતી,
Portuguese,
Ukrainian
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Contributors: Prab R. Tumpati, MD