Riluzole
(Redirected from Tiglutik Kit)
Riluzole is a medication used to treat amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig's disease. It is one of the few treatments available that can extend the life expectancy of patients with ALS. Riluzole works by inhibiting the release of glutamate, a neurotransmitter that is believed to contribute to the degeneration of motor neurons in ALS.
Mechanism of Action[edit | edit source]
Riluzole is classified as a glutamate antagonist. It inhibits the release of glutamate by blocking voltage-gated sodium channels on neurons. This action helps to protect motor neurons from excitotoxicity, a process where excessive glutamate causes neuronal damage and death. By reducing glutamate levels, riluzole helps to slow the progression of ALS.
Indications[edit | edit source]
Riluzole is primarily indicated for the treatment of amyotrophic lateral sclerosis. It is not a cure for ALS but has been shown to extend survival by several months. The medication is typically prescribed to patients who have been diagnosed with ALS and are experiencing symptoms such as muscle weakness and difficulty speaking or swallowing.
Dosage and Administration[edit | edit source]
Riluzole is administered orally, usually in the form of a tablet. The recommended dosage is 50 mg taken twice daily. It is important for patients to take riluzole on an empty stomach, either one hour before or two hours after meals, to ensure proper absorption.
Side Effects[edit | edit source]
Common side effects of riluzole include:
Serious side effects can include liver damage, which is why regular monitoring of liver function tests is recommended during treatment. Symptoms of liver damage may include jaundice, dark urine, and persistent nausea or vomiting.
Contraindications[edit | edit source]
Riluzole is contraindicated in patients with a known hypersensitivity to the drug or any of its components. It should also be used with caution in patients with liver disease or elevated liver enzymes.
Pharmacokinetics[edit | edit source]
Riluzole is rapidly absorbed after oral administration, with peak plasma concentrations occurring within 1 to 1.5 hours. It is extensively metabolized in the liver, primarily by the cytochrome P450 enzyme CYP1A2. The metabolites are then excreted in the urine.
History[edit | edit source]
Riluzole was approved by the Food and Drug Administration (FDA) in 1995 for the treatment of ALS. It was the first drug to be approved for this condition and remains one of the few options available for ALS patients.
Research[edit | edit source]
Ongoing research is exploring the potential use of riluzole in other neurodegenerative diseases, such as Huntington's disease and Alzheimer's disease. Studies are also investigating the combination of riluzole with other therapies to enhance its efficacy in treating ALS.
See Also[edit | edit source]
References[edit | edit source]
External Links[edit | edit source]
| Riluzole | |
|---|---|
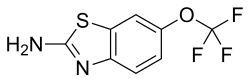
| |
| INN | |
| Drug class | |
| Routes of administration | Oral |
| Pregnancy category | |
| Bioavailability | 60% |
| Metabolism | Liver (CYP1A2) |
| Elimination half-life | 12 hours |
| Excretion | Urine (90%) |
| Legal status | |
| CAS Number | 1744-22-5 |
| PubChem | 5070 |
| DrugBank | DB00740 |
| ChemSpider | 4893 |
| KEGG | D00425 |
Search WikiMD
Ad.Tired of being Overweight? Try W8MD's physician weight loss program.
Semaglutide (Ozempic / Wegovy and Tirzepatide (Mounjaro / Zepbound) available.
Advertise on WikiMD
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Translate this page: - East Asian
中文,
日本,
한국어,
South Asian
हिन्दी,
தமிழ்,
తెలుగు,
Urdu,
ಕನ್ನಡ,
Southeast Asian
Indonesian,
Vietnamese,
Thai,
မြန်မာဘာသာ,
বাংলা
European
español,
Deutsch,
français,
Greek,
português do Brasil,
polski,
română,
русский,
Nederlands,
norsk,
svenska,
suomi,
Italian
Middle Eastern & African
عربى,
Turkish,
Persian,
Hebrew,
Afrikaans,
isiZulu,
Kiswahili,
Other
Bulgarian,
Hungarian,
Czech,
Swedish,
മലയാളം,
मराठी,
ਪੰਜਾਬੀ,
ગુજરાતી,
Portuguese,
Ukrainian
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Contributors: Prab R. Tumpati, MD

