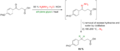
Wolff–Kishner reduction
Wolff–Kishner Reduction is a chemical reaction that involves the complete reduction of a carbonyl group to a methylene group under strongly basic conditions. This reaction is significant in organic chemistry for the modification of ketones and aldehydes into alkanes, without affecting carbon-carbon double or triple bonds. The Wolff–Kishner reduction is particularly useful in synthetic organic chemistry for the preparation of complex molecules.
Overview[edit]
The reaction was independently discovered by Ludwig Wolff and Nikolai Kishner in the early 20th century. It is typically carried out by heating a carbonyl compound with hydrazine (N_2H_4) and a strong base, usually potassium or sodium hydroxide (NaOH or KOH), in a high-boiling solvent such as diethylene glycol. The mechanism involves the formation of a hydrazone, followed by a base-induced elimination of nitrogen, resulting in the formation of an alkane.
Mechanism[edit]
- Formation of Hydrazone: The carbonyl compound reacts with hydrazine to form a hydrazone.
- Base-induced Elimination: The strong base deprotonates the hydrazone, leading to the formation of a nitrogen anion, which then eliminates nitrogen gas, resulting in the formation of a carbanion.
- Protonation: The carbanion is protonated to form the final alkane product.
Variations[edit]
Several modifications of the Wolff–Kishner reduction have been developed to improve its applicability under milder conditions or in specific contexts. These include the Huang-Minlon modification, which is carried out in ethylene glycol at a lower temperature, and the Clemmensen reduction, which is an alternative method for reducing carbonyl compounds using zinc amalgam and hydrochloric acid.
Applications[edit]
The Wolff–Kishner reduction is widely used in the synthesis of complex organic molecules, including natural products and pharmaceuticals. Its ability to selectively reduce carbonyl groups without affecting other functional groups makes it a valuable tool in the chemist's arsenal.
Comparison with Other Reductions[edit]
The Wolff–Kishner reduction is often compared to the Clemmensen reduction, which also reduces carbonyl compounds to alkanes but operates under acidic conditions. The choice between these two methods depends on the sensitivity of the substrate to acidic or basic conditions.
See Also[edit]
- Hydrazine
- Sodium hydroxide
- Potassium hydroxide
- Clemmensen reduction
- Reduction reactions in chemistry
References[edit]
<references/>
-
Wolff–Kishner reduction
-
Wolff–Kishner reduction
-
Wolff–Kishner reduction
-
Wolff–Kishner reduction
-
Wolff–Kishner reduction
-
Wolff–Kishner reduction
-
Wolff–Kishner reduction
-
Wolff–Kishner reduction
-
Wolff–Kishner reduction
-
Wolff–Kishner reduction
-
Wolff–Kishner reduction
-
Wolff–Kishner reduction
Ad. Transform your life with W8MD's Budget GLP-1 injections from $75


W8MD offers a medical weight loss program to lose weight in Philadelphia. Our physician-supervised medical weight loss provides:
- Weight loss injections in NYC (generic and brand names):
- Zepbound / Mounjaro, Wegovy / Ozempic, Saxenda
- Most insurances accepted or discounted self-pay rates. We will obtain insurance prior authorizations if needed.
- Generic GLP1 weight loss injections from $75 for the starting dose.
- Also offer prescription weight loss medications including Phentermine, Qsymia, Diethylpropion, Contrave etc.
NYC weight loss doctor appointmentsNYC weight loss doctor appointments
Start your NYC weight loss journey today at our NYC medical weight loss and Philadelphia medical weight loss clinics.
- Call 718-946-5500 to lose weight in NYC or for medical weight loss in Philadelphia 215-676-2334.
- Tags:NYC medical weight loss, Philadelphia lose weight Zepbound NYC, Budget GLP1 weight loss injections, Wegovy Philadelphia, Wegovy NYC, Philadelphia medical weight loss, Brookly weight loss and Wegovy NYC
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Translate this page: - East Asian
中文,
日本,
한국어,
South Asian
हिन्दी,
தமிழ்,
తెలుగు,
Urdu,
ಕನ್ನಡ,
Southeast Asian
Indonesian,
Vietnamese,
Thai,
မြန်မာဘာသာ,
বাংলা
European
español,
Deutsch,
français,
Greek,
português do Brasil,
polski,
română,
русский,
Nederlands,
norsk,
svenska,
suomi,
Italian
Middle Eastern & African
عربى,
Turkish,
Persian,
Hebrew,
Afrikaans,
isiZulu,
Kiswahili,
Other
Bulgarian,
Hungarian,
Czech,
Swedish,
മലയാളം,
मराठी,
ਪੰਜਾਬੀ,
ગુજરાતી,
Portuguese,
Ukrainian