Triamterene + HCTZ
(Redirected from Dyazide)
What Is Triamterene and Hydrochlorothiazide ?[edit | edit source]
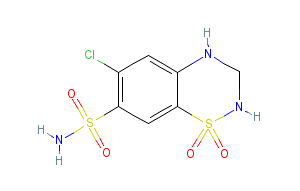
Each triamterene and hydrochlorothiazide capsule for oral use contains triamterene 37.5 mg and hydrochlorothiazide 25 mg. Hydrochlorothiazide is a diuretic/antihypertensive agent and triamterene is an antikaliuretic agent.
What are the uses of this medicine?[edit | edit source]
Triamterene and hydrochlorothiazide capsules, USP are indicated for:
- The treatment of hypertension or edema in patients who develop hypokalemia on hydrochlorothiazide alone.
- For those patients who require a thiazide diuretic and in whom the development of hypokalemia cannot be risked.
- This medicine is used alone or as an adjunct to other antihypertensive drugs, such as beta-blockers. Since triamterene and hydrochlorothiazide capsules, USP may enhance the action of these agents, dosage adjustments may be necessary.
How does this medicine work?[edit | edit source]
- The triamterene and hydrochlorothiazide capsule is a diuretic/antihypertensive drug product that combines natriuretic and antikaliuretic effects.
- Each component complements the action of the other.
- The hydrochlorothiazide component blocks the reabsorption of sodium and chloride ions, and thereby increases the quantity of sodium traversing the distal tubule and the volume of water excreted.
- Hydrochlorothiazide also decreases the excretion of calcium and uric acid, may increase the excretion of iodide, and may reduce glomerular filtration rate.
- The triamterene component of triamterene and hydrochlorothiazide capsules, USP exerts its diuretic effect on the distal renal tubule to inhibit the reabsorption of sodium in exchange for potassium and hydrogen ions.
Who Should Not Use this medicine ?[edit | edit source]
- Triamterene and hydrochlorothiazide capsules, USP should not be given to patients receiving other potassium-sparing agents such as spironolactone, amiloride, or other formulations containing triamterene.
- Potassium supplementation should not be used with triamterene and hydrochlorothiazide capsules, USP except in severe cases of hypokalemia.
- Triamterene and hydrochlorothiazide capsules, USP should not be given to patients with anuria, acute and chronic renal insufficiency or significant renal impairment.
- Triamterene and hydrochlorothiazide capsules, USP should not be used in patients with preexisting elevated serum potassium.
- This medicication should not be given to patients who have Hypersensitivity to either drug in the preparation or to other sulfonamide-derived drugs.
Is this medicine FDA approved?[edit | edit source]
- The combination was approved for medical use in the United States in 1965.
- In 2018, it was the 117th most commonly prescribed medication in the United States, with more than 6 million prescriptions.
How should this medicine be used?[edit | edit source]
- The usual dose of triamterene and hydrochlorothiazide capsules, USP is one or two capsules given once daily, with appropriate monitoring of serum potassium and of the clinical effect.
What are the dosage forms and brand names of this medicine?[edit | edit source]
- Triamterene and Hydrochlorothiazide Capsules, USP contain 37.5 mg triamterene and 25 mg hydrochlorothiazide, and are available.
This medicine is available in fallowing brand names:
- Dyazide® (containing Triamterene, Hydrochlorothiazide)
- Maxzide® (containing Triamterene, Hydrochlorothiazide)
What side effects can this medication cause?[edit | edit source]
Common possible side effects of this medicine include:
- Anaphylaxis, rash, urticaria, subacute cutaneous lupus erythematosus-like reactions, photosensitivity.
- Arrhythmia, postural hypotension.
- Diabetes mellitus, hyperkalemia, hypokalemia, hyponatremia, acidosis, hypercalcemia, hyperglycemia, glycosuria, hyperuricemia, hypochloremia.
- Jaundice and/or liver enzyme abnormalities, pancreatitis, nausea and vomiting, diarrhea, constipation, abdominal pain.'
- Acute renal failure
- Leukopenia, thrombocytopenia and purpura, megaloblastic anemia.
- Muscle cramps.
- Weakness, fatigue, dizziness, headache, dry mouth.
- Impotence, sialadenitis.
What special precautions should I follow?[edit | edit source]
- Caution should be exercised when administering triamterene and hydrochlorothiazide capsules, USP to patients with diabetes, since thiazides may cause hyperglycemia, glycosuria, and alter insulin requirements in diabetes.
- Thiazides should be used with caution in patients with impaired hepatic function.
- Hypokalemia is uncommon with triamterene and hydrochlorothiazide capsules, USP.
- Electrolyte imbalance, often encountered with this therapy with triamterene and hydrochlorothiazide capsules, USP when using high doses for prolonged periods or in patients on a salt-restricted diet.
- Triamterene and hydrochlorothiazide capsules, USP should be used with caution in patients with a history of renal stones.
What to do in case of emergency/overdose?[edit | edit source]
The signs and symptoms of overdosage(Electrolyte imbalance) include:
- polyuria
- nausea
- vomiting
- weakness
- lassitude
- fever
- flushed face
- hyperactive deep tendon reflexes
- If hypotension occurs, it may be treated with pressor agents such as levarterenol to maintain blood pressure.
- Induce immediate evacuation of the stomach through emesis or gastric lavage.
- There is no specific antidote.
Can this medicine be used in pregnancy?[edit | edit source]
- The routine use of diuretics in an otherwise healthy woman is inappropriate and exposes mother and fetus to unnecessary hazard.
- Diuretics do not prevent development of toxemia of pregnancy, and there is no satisfactory evidence that they are useful in the treatment of developed toxemia.
What should I know about storage and disposal of this medication?[edit | edit source]
- Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
- Protect from light.
- Dispense in a tight, light-resistant container as defined in the USP, with a child-resistant closure.
| Diuretics (C03) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Search WikiMD
Ad.Tired of being Overweight? Try W8MD's physician weight loss program.
Semaglutide (Ozempic / Wegovy and Tirzepatide (Mounjaro / Zepbound) available.
Advertise on WikiMD
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Translate this page: - East Asian
中文,
日本,
한국어,
South Asian
हिन्दी,
தமிழ்,
తెలుగు,
Urdu,
ಕನ್ನಡ,
Southeast Asian
Indonesian,
Vietnamese,
Thai,
မြန်မာဘာသာ,
বাংলা
European
español,
Deutsch,
français,
Greek,
português do Brasil,
polski,
română,
русский,
Nederlands,
norsk,
svenska,
suomi,
Italian
Middle Eastern & African
عربى,
Turkish,
Persian,
Hebrew,
Afrikaans,
isiZulu,
Kiswahili,
Other
Bulgarian,
Hungarian,
Czech,
Swedish,
മലയാളം,
मराठी,
ਪੰਜਾਬੀ,
ગુજરાતી,
Portuguese,
Ukrainian
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Contributors: Prab R. Tumpati, MD